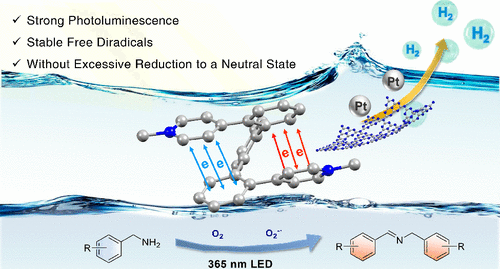
A series of novelortho-terphenylene viologen derivatives (o-TPV2+) with through-space conjugation (TSC) via the combination ofortho-terphenylene skeletons with viologen structure is reported. Their optoelectronic properties can be adjusted byN-arylation orN-alkylation reactions. Compared with other viologen derivatives,o-TPV2+not only exhibits strong photoluminescence but also retards the charge recombination process and stabilizes the diradical state without forming a quinoid structure due to the special TSC effect. Based on their special redox characteristics,o-TPV2+was applied to the photocatalytic oxidative coupling of benzylamine with 96% yield. In addition,pTA-o-TPV2+(tethered withp-toluic acid)-modified g-C3N4was used for visible-light-driven hydrogen production for the first time, exceeding 15 times the rate over unmodified g-C3N4.
原文链接:https://pubs.acs.org/doi/10.1021/jacs.1c11577