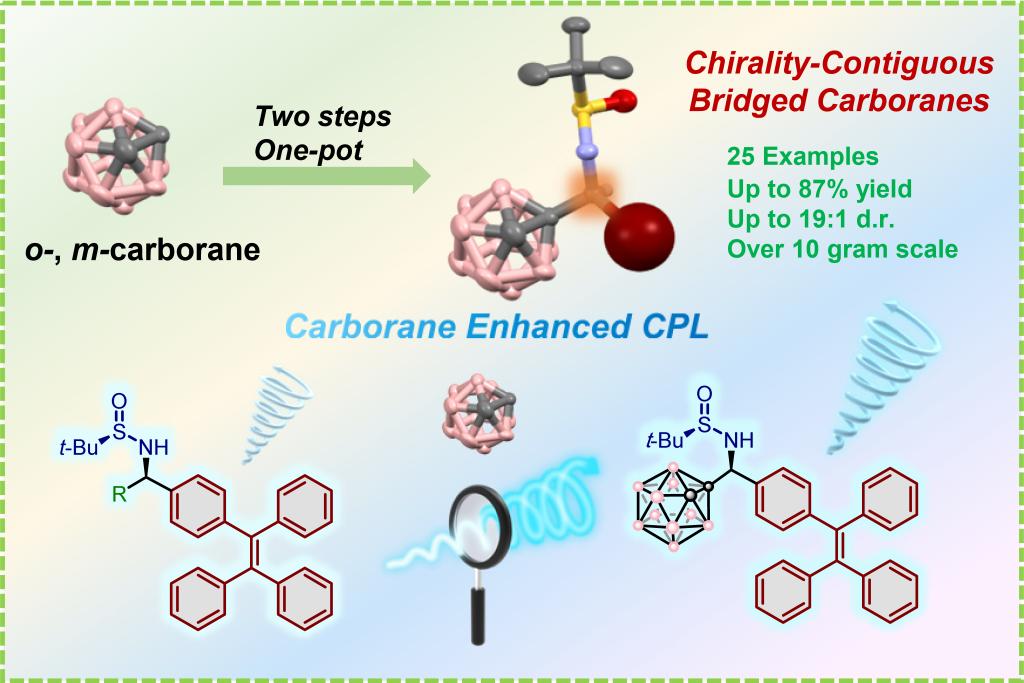
Icosahedral carboranes are promising structural motifs for optoelectronic functional materials owing to their unique three-dimensional aromatic architectures and distinctive electronic properties. However, their applications remain restricted by the absence of efficient and scalable asymmetric synthesis and limited circularly polarized luminescence (CPL) studies. Herein, we developed a highly stereoselective and straightforward asymmetric synthesis for chiral carborane molecules. This method employs readily available starting materials and enables the precise construction of optically pure chirality-contiguous bridged carborane derivatives via a two-step, one-pot protocol, achieving scalable synthesis on a 10 g-scale without chiral HPLC resolution. After chiral carborane substituents were introduced onto the organic conjugated chromophores, a series of CPL-active molecules with excellent performance were obtained. Their luminescence dissymmetry factors (glum) reached up to 10–3level, and the quantum yields (ΦPL) achieved up to 0.70. Furthermore, it has been observed that the introduction of carborane into the chiral bridging structure significantly enhances theΦPLandglumvalues of the luminescent compounds. Such concurrent enhancement of both parameters is scarcely documented. Applying one of the chiral emissive compounds, a good enantioselective discrimination of several free amino acids in the aqueous phase has been achieved. This study provides an effective and feasible synthetic route for the stereoselective large-scale synthesis of diverse chiral carborane derivatives. The demonstrated enhancements in both photophysical metrics and chiral recognition applications highlight the dual functionality of these hybrid systems, providing strategic insights for developing chiral materials in polymer science, pharmaceutical chemistry, and optoelectronics.
原文链接:https://pubs.acs.org/doi/10.1021/jacs.5c03381